18. The dissociation constant for n acid indicates the extent to which it dissolves into irons. The higher of dissociation constant, the stronger the acid.
The dissociation constant for some acids is given below along with two possible correct statements.
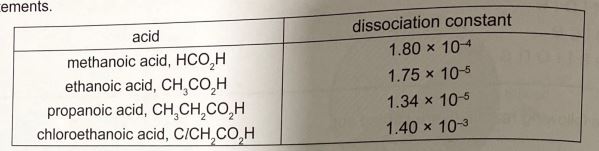
Statement 1: Increasing the length of the carbon chain makes the acid stronger.
Statement 2: Replacing the hydrogen by a chlorine in ethanoic acid makes the acid stronger.